How Many Sigma and Pi Bonds in a Double Bond
The pi bond is not as strong as the sigma bond and the electron cloud above and below the plane is polarizable. Other carbon compounds and other molecules may be explained in a similar way.

Sigma And Pi Bonds Brilliant Math Science Wiki
The strength disparity is determined by the number of component bonds.
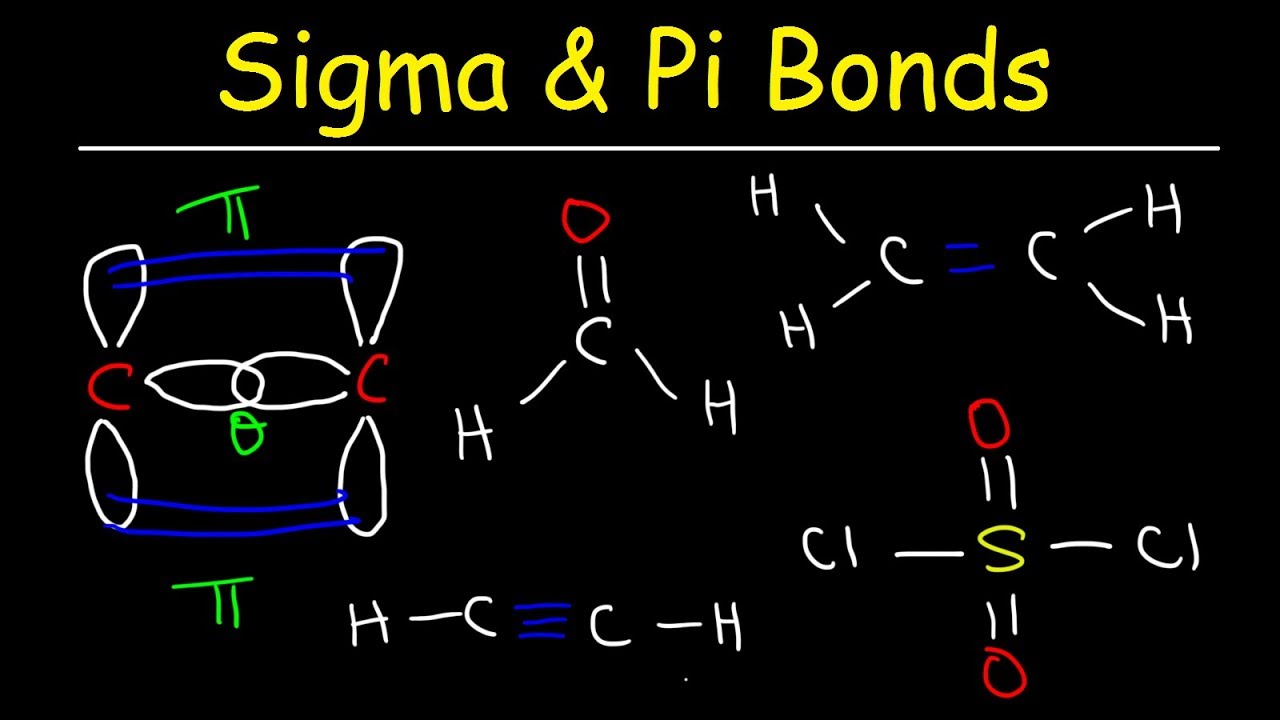
. 2 sigma and 2 pi bonds. For example ethene C 2 H 4 has a double bond between the carbons. This can be illustrated by comparing two types of double bonds one polar and one nonpolar.
Pi bonds are usually weaker than sigma bondsThe C-C double bond composed of one sigma and one pi bond has a bond energy less than twice that of a C-C single bond indicating that the stability added by the pi bond is less than the stability of a sigma bond. Sp hybridization in acetylene corresponds with two sigma. 2 sigma and one pi bond.
The sigma bond has similar properties to those found in alkanes while the pi bond is more reactive. The hydroxyl oxygen allows one of its lone pair electrons to conjugate with the pi system of the carbonyl group. For this molecule carbon sp 2 hybridises because one π pi bond is required for the double bond between the carbons and only three σ bonds are formed per carbon atom.
As seen in above table 1 a regular carbon-carbon single bond is 347 kJmol whereas in a carbon-carbon double bond the pi bond rises the bond strength by 267 kJmol. It is not easy to flip between the two. How is a pi bond formed.
A typical example of this mechanism is the addition of hydrogen halides where proton from strong acid may yield a carbocation. The oxygen atom of the carbonyl group and that of the hydroxyl group has two lone pairs each. 1 sigma and 1 pi bond.
A pi bond is not an axial bond. Also there are many non-benzene kinds of aromatic compounds existing too. Indicate the number of sigma bonds and the number of pi bonds in the molecule CO32-.
The sigma bond in the CC for ethene forms between two sp2 hybrid orbitals of two carbon atoms and a pi bond for between two p orbitals. In the living organisms take for example the very common type of aromatic ring is the DNA and RNA bases with double chains. Adding an additional pi bond causes a more increase of 225 kJmol.
A double bond on the other hand is made up of one sigma bond and one pi bond while a triple bond is made up of one sigma bond and two pi bonds. The word aromatic in the real sense refers to the benzene derivatives and as it was defined the way first. The 2s orbital mixes with only two of the three available 2p orbitals.
From knowing the hybridization of the central atom we can determine the number of sigma bonds around the central atom but no more than that without more information. In any multiple bonds there will be one sigma bond and the remaining one or two bonds will be pi bonds. One pure double bond has one sigma and one pi bond and one pure triple bond has one sigma and two pi bonds.
Rotation around the double bond is disfavored so alkenes form fairly stable isomers depending on the positioning of substituents on the same cis or opposite. View Answer The bond dissociation energy is the amount of energy required to break a bond a so as to produce. The diboron bond which is a pi bond is an exception.
Now that we understand the difference between sigma and pi electrons we remember that the pi bond is made up of loosely held electrons that form a diffuse cloud which can be easily distorted. The formation of 3 sigma bonds gives the carbonyl group a basic trigonal shape with bond angles of 120 degrees. The CC double bond on the left below is nonpolar.
Pi bonds are formed from the sideways overlap of. The carbon atoms in the double bond are sp 2 hybridized forming a planar structure. C-C sigma bond C-C pi bond C C Cl H H Cl C C Cl H Cl H This.
Electrophilic addition reactions of alkenes Addition of hydrogen halides. In sp 2 hybridisation the 2s orbital is mixed with. Single carbon-carbon covalent bonds can easily rotate All three of these structures are the same CC double bonds have restricted rotation due to position of the pi bond so the groups on either end of the bond are fixed in one position.
1 sigma and 2 pi bonds. Because the single bond has only a sigma bond it is the weakest of the. From the perspective of quantum mechanics this bonds weakness is explained by significantly less overlap between.
If a bond between two atoms is broken when one atom is rotated around the bond axis that bond is called a pi bond. In an ethene molecule a double bond between carbons forms with one sigma and one pi bond. This way a double bond can act as a nucleophile.

14 1 Sigma And Pi Bonds Hl Old Version Youtube

Sigma And Pi Bonds Explained Basic Introduction Chemistry Youtube
How Many Sigma And Pi Bond Count Number Of Sigma And Pi Bonds Example Practice Problem Shortcut Youtube

0 Response to "How Many Sigma and Pi Bonds in a Double Bond"
Post a Comment